Lewis Acid/Base Theory
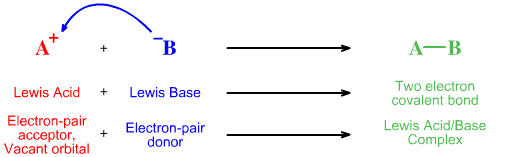
A Lewis base is a species with an available (reactive) pair of electrons and a Lewis acid is an electron pair acceptor. The simplest reaction is for a Lewis acid to interact with a Lewis base to give a Lewis acid/base complex:
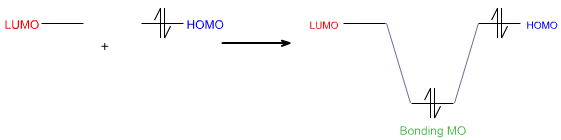
Lewis Acid + Lewis BaseIn modern theoretical language, the Lewis acid's LUMO – its Lowest Unoccupied Molecular Orbital – interacts with the Lewis base's HOMO – its Highest Occupied MO – to give a bonding molecular orbital.Lewis Acid/Base Complex
LUMO + HOMOLewis acids are often said to have a vacant orbital.Bonding MO
The simplest Lewis acid plus Lewis base interaction is complexation and this process can be represented using both Lewis theory and FMO theory:
Using Lewis theory, electron accountancy and curly arrows, the lone-pair of electrons moves from the Lewis base to the Lewis acid, the electron-pair acceptor, to give a two electron chemical bond. The curly arrow represents the movement of the electron-pair:
Using FMO theory, the Lewis base's highest occupied molecular orbital or HOMO interacts with the Lewis acid's lowest unoccupied molecular orbital or LUMO to give a bonding molecular orbital:
In The Reaction Flask
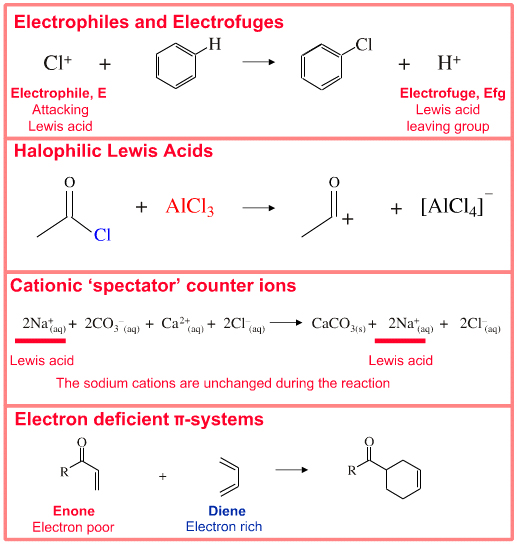
In the test tube we experience many types of reaction that we explain in terms of Lewis acid/base interactions, including: anions & cations in solution, lone-pairs, ligands, spectator ions, nucleophiles, nucleofuges, electrophiles, electrofuges, ionic substitution, addition, elimination & rearrangement, precipitates, Brønsted acids, proton accepting bases, transition metal complexes, cycloaddition, and more.Classifying Lewis acid behaviour: All species with an electron pair accepting (vacant) orbital. All species with full or partial positive charge behave as Lewis Acids. Lewis Acid behaviour is found amongst:
• Metal cations complexed by ligands
• Electrophiles (attacking Lewis acids)
• Electrofuges (Lewis Acid leaving group)
• Classic electron deficient species such as BF3 and AlCl3
• Cationic spectator counter ions
• Electron deficient π-systems which take part in multicentre interactions
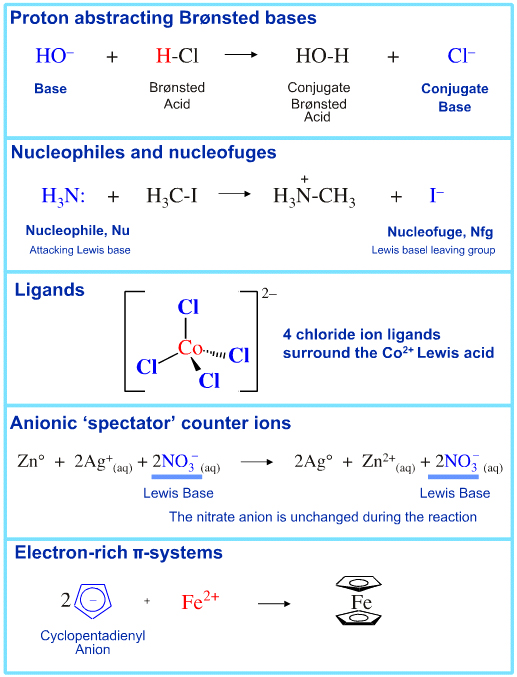
Lewis Base species have a pair of electrons to donate, or an available HOMO. All species with full or partial charge behave as Lewis Bases. Lone-pair donation behaviour is found amongst:
• Anions
• Proton abstractors
• Conjugate Brønsted bases
• Nucleophiles
• Nucleofuges
• Ligands
• Anionic counter ions
• Electron-rich π-systems
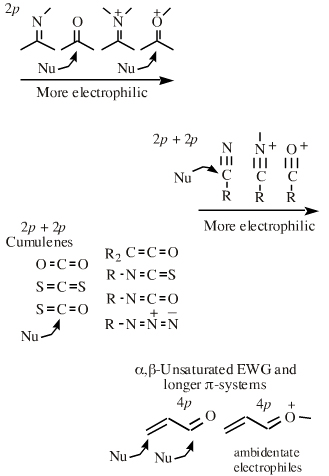
Neutral species with polarised bonds (methyl iodide, carbonyl functions, etc.) behave as if they have Lewis acid and Lewis base ends or poles. The differentiation becomes more pronounced as bond polarisation increases. Thus, the carbon atoms of both methyl iodide and a carbonyl function are made delta+ by the electronegative iodine and oxygen atoms.
The delta+ carbon atoms of both methyl iodide and carbonyl functions are susceptible to attack by nucleophilic Lewis bases.
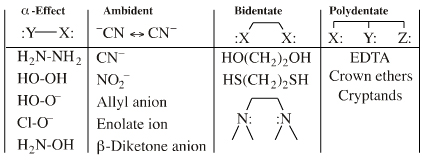
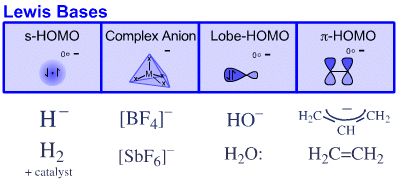
Four types of Lewis base are recognised:
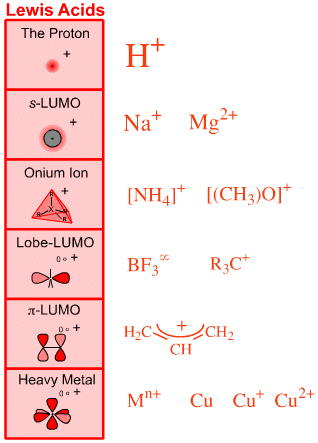
s-HOMO Lewis basesSix types of Lewis acid are recognised:
Hydride ion, H–, and hydrogen, H2
Complex Anion Lewis bases
Tetrafluoroborate ion, [BF4]–
Lobe-HOMO Lewis bases
Hydroxide ion, HO–, water, H2O:, methylcarbanion, H3C–, etc.
π–HOMO Lewis bases
Electron rich π-systems: ethene, benzene, etc.
The Proton Lewis acid
The proton, H+
s-LUMO Lewis acids
Group 1 and 2 cations: Li+, Mg2+, etc.
Onium Ion Lewis acids
Ammonium ion, [NH4]+, oxonium ion, [OH3]+, etc.
Lobe-LUMO Lewis acids
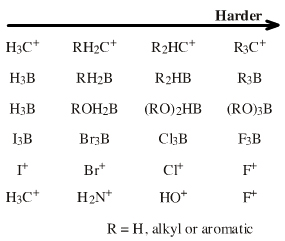
Boron trifluoride, BF3, the carbenium ion, H3C+
π-LUMO Lewis acids
Electron poor π-systems: enones, tetracyanoethylene, etc.
Heavy Metal Lewis acids
Cations and bulk metals of the: transition metals, post-transition metals, lanthanides and actinides
Lewis acids & Bases in Detail
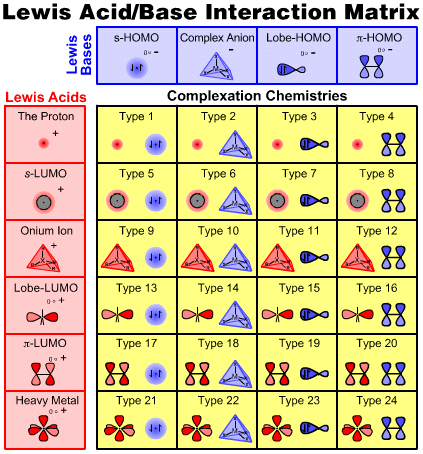
The six types of Lewis acid and four types of Lewis base can be arranged into an interaction matrix that generates 24 types of Lewis acid/base interaction complex and associated reaction chemistry, as discussed in detail on the next page of this webbook.s-HOMO Lewis Bases
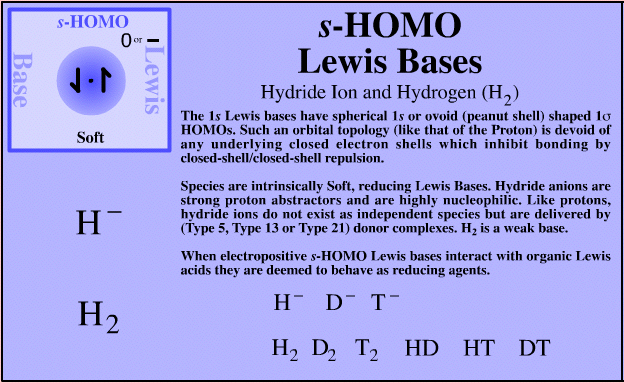
| Hydride Ion, Hydrogen & Helium | H– D– T– H2 D2 T2 HD HT DT He Search for s-HOMO Lewis bases species in The Chemical Thesaurus |
| FMO Topology: | s-HOMO Lewis bases have spherical 1s or ovoid (peanut) 1s HOMOs which are devoid of the closed electron shells which hinder complexation (due to closed-shell/closed-shell repulsion) seen in all other Lewis base types. |
| Charge: | Negative (H–, hydride) or neutral (H2, hydrogen). |
| HSAB: | Intrinsically soft. The softest and most polarisable of all species. |
| Chemistry: | Nearly all of the elements are able to form hydride compounds: When s-HOMO Lewis bases interact with Lewis acids to form a complex they are deemed to reduce the Lewis acid: s-HOMO Lewis bases are reducing agents. The hydride ion is a strong proton abstracting base and nucleophile. H2 is only very weakly basic such that H2, D2, T2, HD, HT and DT hardly seem to be basic at all, yet as they can be protonated to [H3]+ (in the gas phase) they must be Lewis bases. H– and H2 form metallic complexes. Helium can be protonated under gas phase conditions to [HHe]+ so it is a Lewis base, an s-HOMO Lews base. |
| Congeneric Series: | H– D– T– H2 D2 T2 HD HT DT |
Complex Anion Lewis Bases
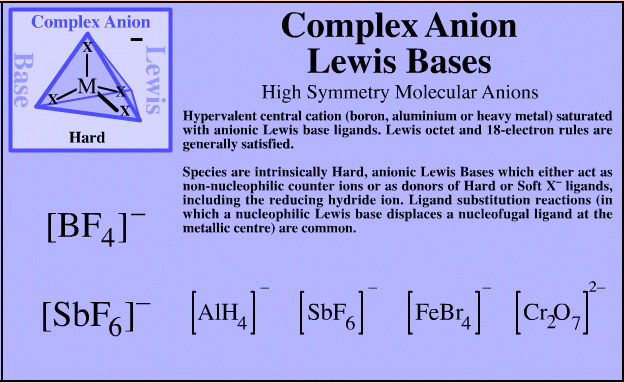
| High Symmetry Molecular Anions | 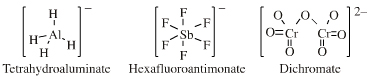 Search for complex anion Lewis bases species in The Chemical Thesaurus |
| FMO Topology: | Complex anion Lewis bases have a hypervalent central cation (boron, aluminium or heavy metal) saturated with anionic Lewis base ligands. Lewis octet and 18-electron rules are generally satisfied. The HOMO shows high spherical symmetry. |
| Charge: | Negative. |
| HSAB: | Intrinsically hard. |
| Chemistry: | Complex anion Lewis base species behave as charged hard spheres that form ionic charge-controlled complexes (ie act as non-nucleophilic counter ions), or they behave as donors of hard/soft ligands, X–. Ligand substitution – in which a nucleophilic Lewis Base displaces a nucleofugal ligand – is common and ligand symbiosis considerations/effects are very important. There are four subclasses of complex anion Lewis base: X = Halogen anion which gives rise to the synthetically useful non-basic, non-nucleophilic, non-interfering anionic spectator counter ions: [BF4]– [SbF6]– [AsF6]– [FeBr4]–X = Hydride ion which gives rise to species which act as donors of nucleophilic hydride ion, [BH4]– and [AlH4]–, as long as there is not a Brønsted Acidic proton available or H2 is generated. M = Heavy metal (Fe or Cr as opposed to B or Al). Such complex anions are much studied in classical inorganic coordination chemistry. Transition metals centres often exhibit multiple oxidation states. These are better considered as type 23 Lewis acid/base complexes. X = Oxygen heavy metal species with oxygen ligands are commonly used as oxidising agents. |
| Congeneric Series: | Families of ligand replacement congeneric series are common: 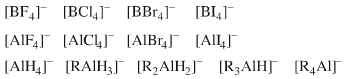 |
Lobe-HOMO Lewis Bases
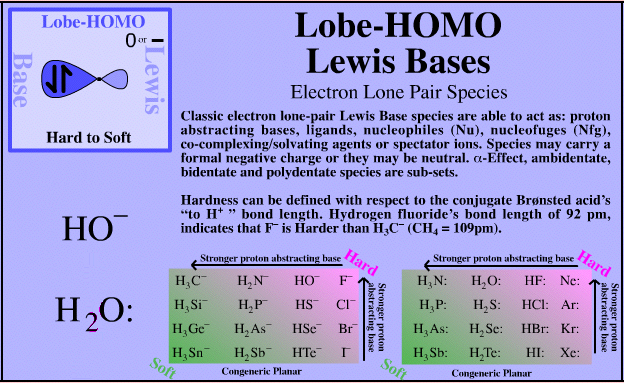
| Electron Lone Pair Species | 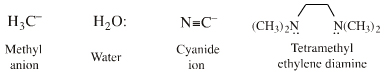 Search for Lobe-HOMO Lewis base species in The Chemical Thesaurus |
| FMO Topology: | Typical electron lone pair species in which the Lewis base HOMO is a px FMO orbital. In valence bond terms, the electron pair is in an sp3, sp2 or sp hybrid orbital. On complexation species show directional bonding which implies that the HOMO is directional, ie lobe shaped. |
| Charge: | Negative or neutral with a lone-pair of electrons. |
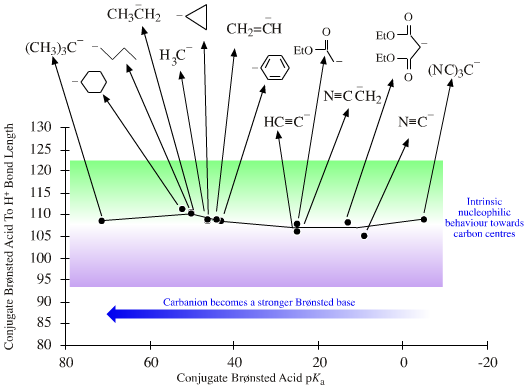
| HSAB: | Species range from hard to soft. The hardness of anionic Lobe-HOMO Lewis bases can be defined with respect to the methyl anion, H3C– the carbon-hydrogen bond length in methane (109pm). All Lobe-HOMO Lewis bases can by definition be protonated and the conjugate Brønsted acid's proton-to-Lewis base bond length, along with its pKa value, serves to probe the Lewis base's chemistry and behaviour. Bond-length and pKa data are linear over congeneric series and planars. Methane, CH4, is a convenient reference Lobe-HOMO Lewis base due to the importance of carbon in organic chemistry and that so many reactions occur at carbon centres. Congeneric species with base-to-H+ bond lengths shorter than 109pm, such as the hydroxide ion HO– (HO–H bond length = 96pm), are deemed to be harder than the methyl carbanion. Longer bond-length as seen with iodide, I–, (HI bond length = 161pm) equates with softness. |
| Chemistry: | Lobe-HOMO Lewis bases are the classic electron lone-pair donor species that can act as:
The atomic lone pair centre may be embedded in a π-system, for example the allyl ion, in which case the species can be dual classified as a Lobe-HOMO and a π-HOMO Lewis base. When the species reacts via a single atomic centre it is classified as a Lobe-HOMO Lewis base. There are several subclasses of Lobe-HOMO Lewis bases, including:
 |
| Congeneric Series: | There are two important congeneric planars to be found within electronegative main group elements and their anions. One is formed by the X– anions and the other by X: neutral lone pair species. Read more about the chemistry of these congeneric planars elsewhere in this webbook, here: 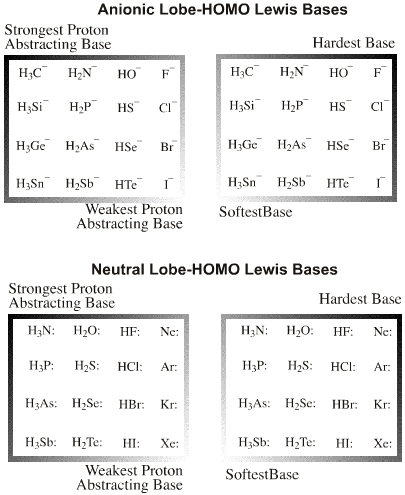 |
| The Inerts | A number of Group 14 elemental hydrides: CH4, SiH4, GeH4& SnH4, are rather inert towards Lewis acid and Lewis base reagents. (Species can be oxidised and they are susceptible to attack by radicals and diradicals.) However, methane can be protonated by super acids to the carbonium ion: H+ + CH4 –> [CH5]+So methane is a Lewis base but, like helium, it is an exceedingly feeble proton abstractor. It follows that: CH4, SiH4, GeH4, SnH4 and related compounds are Lewis bases. Search for inert species in The Chemical Thesaurus |
π-System Lewis Bases
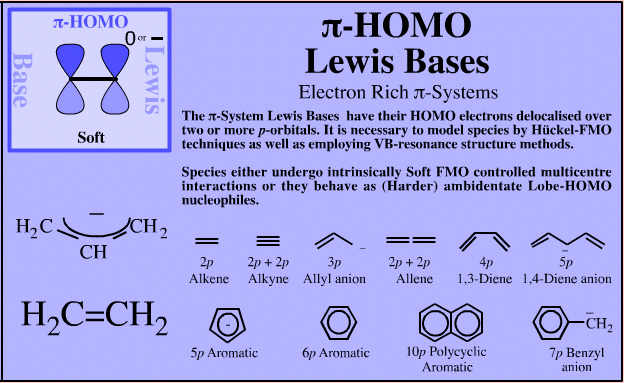
| Electron Rich π-Systems | 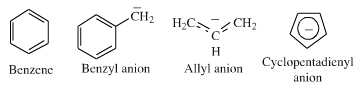 If an extended electron rich π-system species reacts via a single atomic centre, for example when an allyl anion is protonated to give propene, the species is better considered behaving as an ambidentate, π-stabilised Lobe-HOMO Lewis base. However, when when the species reacts via its extended π-system directly, for example during Diels-Alder cycloaddition or when forming a π-organometallic complex, the the species should be considered as a π-HOMO Lewis base species. Thus, there is an overlap between π-stabilised Lobe-HOMO and π-HOMO classification. Search for π-HOMO Lewis base species in The Chemical Thesaurus |
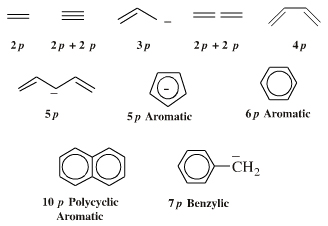
| FMO Topology: | π-HOMO Lewis bases have their electrons in their highest occupied molecular orbital, or HOMO, delocalised over two or more p-orbitals. Species require modelling by Hückel-FMO techniques as well as by VB-resonance structure methods. Hückel MO modelling gives rise to whole families of π-structure: polyene ribbons, aromatics, etc.: Indeed, quantum mechanics is all about patterns. A particularly striking manifestation is seen with the polyene system of: 1, 2, 3, 4, 5, 6... conjugated p-orbital systems and how they give rise to the carbanion, allyl anion & pentatrieneyl anion and alkene, 1,3-diene & 1,3,5-triene π-HOMO Lewis bases: 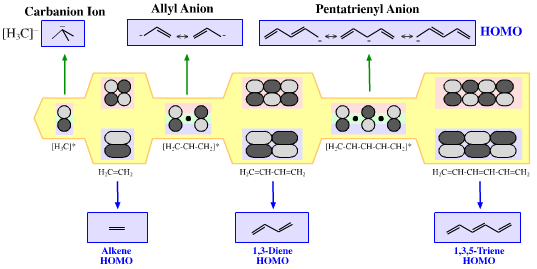 |
| Charge: | Negative, delta– or electron rich π-systems. |
| HSAB: | Soft when the entire π-system is acting as the Lewis base, but harder when a single atomic centre is involved and the species is behaving as a Lobe-HOMO Lewis base. The allyl anion can behave as (or be considered as) 2 π-electrons delocalised over a 3p orbital function, or as a stabilised 2p orbital carbanion. In this latter case it is better to consider the allyl anion to be behaving as a (harder) Lobe-HOMO Lewis base. |
| Chemistry: | Species behave as π-species when they undergo FMO controlled multicentre interactions. These most obviously manifest themselves in three situations:
|
| Congeneric Series: | 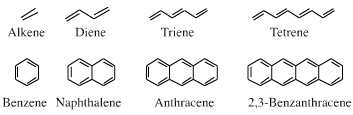 |
The Proton Lewis Acid
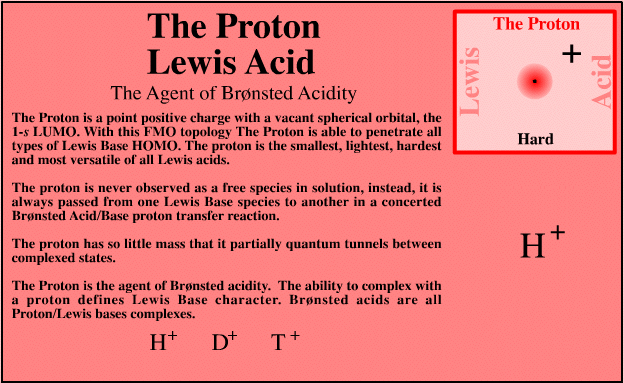
| The Proton | Search for proton Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | The proton is a point positive charge with a vacant spherical orbital, the 1s LUMO. This geometry enables the proton to penetrate all types of Lewis base HOMO topology. |
| HSAB: | Intrinsically very hard |
| Chemistry: | The proton is the smallest, lightest, hardest and most versatile Lewis acid. However, the proton is never observed free (in chemistry at least, high energy high vacuum physics is different). The proton is always passed or transferred from one Lewis base to another in a concerted Brønsted acid/base proton transfer reaction. The proton has so little mass that it (partially) quantum tunnels between complexed states, and the ability of a species to complex with a proton defines Lewis base character. Brønsted acids are all proton/Lewis bases complexes: the proton is the agent of Brønsted acidity.The Ka and pKa of are a measure of Brønsted acid strength with respect to water. As the Lewis acid H+ remains constant, the terms Ka and pKa are a measure of a conjugate (Lewis) base's affinity for H+ with respect to the standard Lewis base water, :OH2. |
| Congeneric Series: | H+ D+ T+ |
s-LUMO Lewis Acids
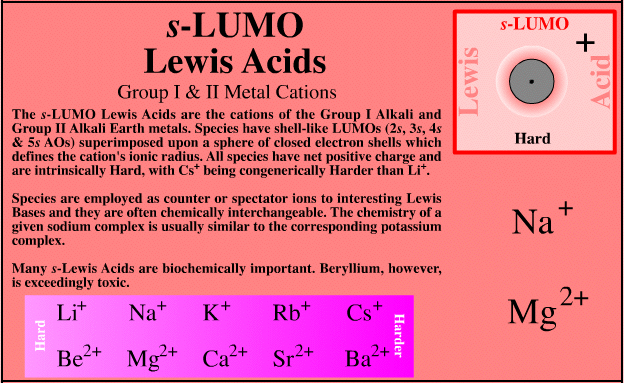
| Group I & II Metal Cations | 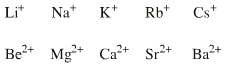 Search for s-Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | The s-Lewis acids are the cations of the Group I alkali and Group II alkaline earth metals. The shell-like LUMO (2s, 3s 4s 5s & 6s AOs) is superimposed upon a sphere of closed electron shells which defines the ionic radius of the cation. |
| Charge: | Positive |
| HSAB: | Intrinsically hard. Fajan's rules indicate that small highly charged cations, for example Be2+, are able to polarise anions and give polar covalent complexes. |
| Chemistry: | Used as counter ions or spectator ions to interesting Lewis bases. Very important biochemical species. |
| Congeneric Series: | There are two s-LUMO series: Group I alkali metals: Li+ Na+ K+ Rb+ Cs+ Group II alkali earth metals: Be2+ Mg2+ Ca2+ Sr2+ Ba2+ |
Onium Ion Lewis Acids
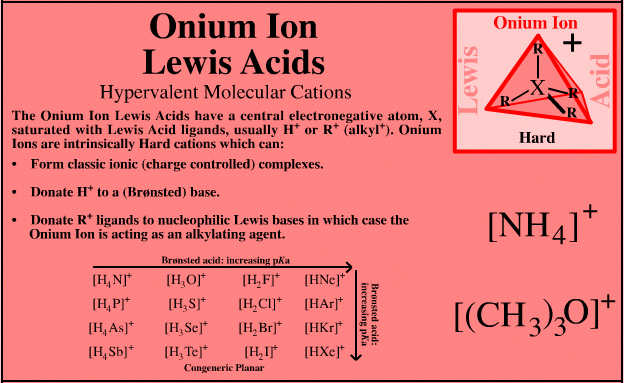
| Hypervalent Molecular Cations | 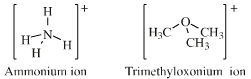 Search for onium ion Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | The onium ion Lewis acids have a central electronegative atom saturated with Lewis acid "ligands", usually H+ or alkyl+. Onium ion Lewis acids are all proton/X Lobe-HOMO or carbenium ion/X Lobe-HOMO complexes where: X = N, O, F, Ne, P, S, Cl, Ar, As, Se, Br, Kr, Sb, Te, I, Xe |
| Charge: | Positive |
| HSAB: | Intrinsically hard, but species behave as a source of hard H+ or the relatively soft Lobe-LUMO Lewis acid carbenium ion, H3C+. |
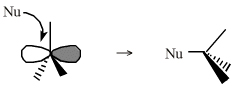
| Chemistry: | Onium ions either form charge-controlled (ionic) complexes or they react by transferring a ligand to a nucleophilic /basic Lewis base. If the transferred ligand is H+, the onium ion acts as a Brønsted Acid. If the transferred ligand is a carbenium ion Lewis acid, the onium ion is said to be an alkylating agent. High symmetry tetraalkyl ammonium ions, such as [(CH3)4N]+, can act as spectator cations. Methane can be protonated to the five valent carbonium ion: [CH5]+ Second order nucleophilic substitution reactions at carbon pass through a five valent carbonium ion transition state: |
| Congeneric Series: | There is one onium ion Lewis acid planar: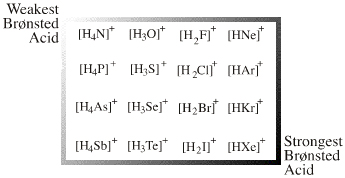 Ammonium, phosphonium, oxonium and sulfonium ions give rise to many ligand replacement congeneric series, for example: 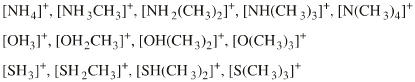 [R4N]+ [R3NR']+ [R2NR2]+ [RNR'3]+ [NR'4]+ where R and/or R' = H, CH3, alkyl, C6H5 etc. |
Lobe-LUMO Lewis Acids
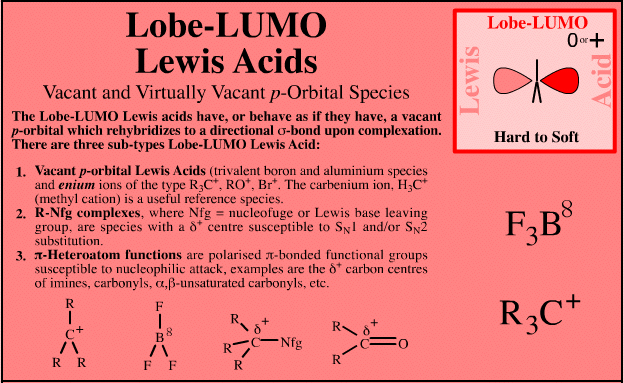
| Vacant p-Orbital Species | 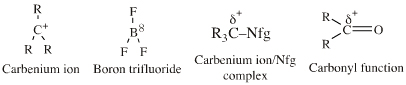 Search for lobe-LUMO Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | Lobe-LUMO Lewis acid species either have a vacant p orbital (R3C+ or F3B), or they have an important resonance structure (ie a 'mixed-in' LUMO) which gives the species considerable vacant p orbital character. Such Lobe-LUMO centres are polarised delta+. |
| Charge: | Positive or delta+. |
| HSAB: | Hard to soft. Hardness of Lobe-LUMO Lewis acids is here defined with respect to the carbon-hydrogen bond length in methane (109pm). All Lobe-LUMO Lewis acids can complex with hydride ion and the corresponding Type 11 Complex's Lewis acid to hydride bond length serves to probe the Lewis acid's chemistry. It transpires that bond-length data is linear along congeneric series and over planars. It is convenient to nominate methane as a reference Lobe-LUMO Lewis acid because of its importance in organic chemistry. Methane can be deconstructed to the carbenium ion Lobe-LUMO Lewis acid and a Hydride ion Lewis base. The H3C+-to-H– bond length, ie methane's C-H bond length of 109pm, can be used as a reference point with which to compare to other Lobe-LUMO Lewis acids. Congeneric species with 'Lewis acid-to-H–' bond lengths shorter than 109pm (such as the hydroxy cation HO+, HOH bond length = 96pm) are deemed to be harder than the carbenium ion. Many Lobe-LUMO Lewis acids react via concerted SN2 mechanisms. These reactions exhibit transition state symbiosis. Hard nucleofuges, such as fluorosulfonate FSO2O–, render the Lobe-LUMO centre harder, and soft nucleofuges, such as iodide ion I–, render the centre softer. |
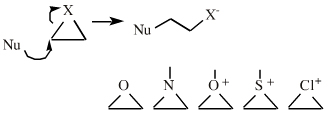
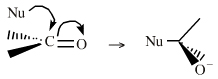
| Chemistry: | Species are susceptible to attack by nucleophilic Lewis bases and they may be actively electrophilic. Lobe-LUMO Lewis acids increase the extent of the sigma-skeleton when they complex with nucleophiles. There are three subclasses of Lobe-LUMO Lewis acids. Members of each subclass have the property that they complex with, or are attacked by, nucleophilic Lewis bases. Vacant p-orbital Lewis Acids Trivalent boron and aluminium species, BF3 and AlCl3, and enium ions of the type R3C+, RO+, Br+. The methyl cation carbenium ion, H3C+, is a useful reference species. Species undergo A + B -> A-B complexation reactions, where B is a nucleophile, Nu: There are several congeneric series: And many congeneric dots: R-Nfg Complexes Where Nfg = nucleofuge or Lewis base leaving group. Species susceptible to SN1 and SN2 nucleophilic substitution: H3C-I, (H3C)3C-Cl, H3C-OTs, epoxides etc. The (usually carbon) centre attached to the nucleofuge is rendered delta+: Alkyl groups stabilise the carbenium ion centre: 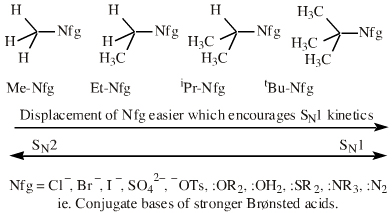 There quite a number of nucleofugal leaving groups, including halide ions, sulfate, tosylate, triflate, etc. The liability of a leaving group – how easily it is displaced – correlates with the pKa of Lewis base's conjugate acid. Thus, an Nfg with a strong conjugate Brønsted acid, such as bromide ion (HBr) is a good leaving group and is easily displaced. Further examples are found with three membered rings with an O, N, S, etc. heteroatom, and that are susceptible to nucleophilic attack and ring opening: π-Heteroatom Functions Polarised π-bonded functional groups susceptible to nucleophilic addition, or nucleophilic addition-followed-by-elimination, which leads to net substitution. The delta+ carbon centres of imines, carbonyls, alpha,beta-unsaturated carbonyls, etc.: |
| Congeneric Series | A rich source of congeneric series. |
π-LUMO Lewis Acids

| π-LUMO Lewis Acids | 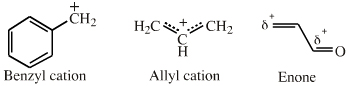  If an extended electron poor π-system species reacts via a single atomic centre, for example when a benzyl cation is reduced to toluene, the species is better considered behaving as an ambidentate, π-stabilised Lobe-LUMO Lewis acid. However, when when the species reacts via its extended π-system directly, for example during Diels-Alder cycloaddition or when forming a π-organometallic, the the species should be considered as a π-HOMO Lewis base species. Thus, there is an overlap between π-stabilised Lobe-LUMO and π-LUMO classification. Search for π-LUMO Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | Delocalised cationic hydrocarbon π-systems and those neutral but electron deficient π-functions which participate in concerted multicentre reactions. Hückel MO theory gives rise to whole families of π-structure: polyene ribbons, aromatics, etc. Each system is FMO unique. π-Species must be considered at the Hückel level, as well as by VB resonance techniques. Indeed, quantum mechanics is all about patterns. A particularly striking manifestation is seen with the polyene system of: 1, 2, 3, 4, 5, 6... conjugated p-orbital systems and how they give rise to the cabenium ion, allyl cation & pentatrieneyl cation and alkene, 1,3-diene & 1,3,5-triene π-LUMO Lewis acids: 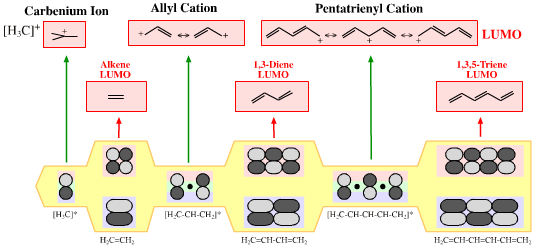 |
| Charge: | Positive or delta+ electron poor π-systems. |
| HSAB: | Intrinsically soft. |
| Chemistry: | Species behave as π-LUMO Lewis acid species when they undergo FMO controlled multicentre interactions. These most obviously manifest themselves in three situations:
|
| Congeneric Series: | Few congeneric series, but the chloronitrobenzene series can be considered congeneric with respect to the nucleophilic displacement of Cl– by a nucleophile: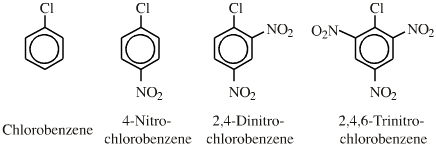 |
Heavy Metal Lewis Acids

| Transition, Post-Transition, Lanthanide & Actinide Cations & Bulk Metals | 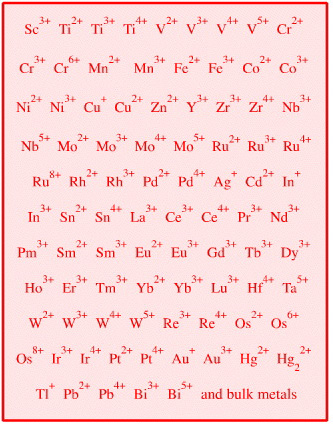 Search for heavy metal Lewis acid species in The Chemical Thesaurus | |||||||||
| FMO Topology: | Multiple vacant lobe-shaped p, d or f orbitals which may rehybridize on complexation. Many orbitals available for back-bonding. Pearson's original analysis remains excellent. | |||||||||
| Charge: | Positive or neutral. | |||||||||
| HSAB: | Hard to soft. Pearson states in his early HSAB publications, see elsewhere in this webbook, that transition metal ions of high oxidation state are harder than those of low oxidation state.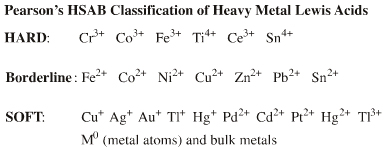 | |||||||||
| Chemistry: | Heavy metals exhibit variable oxidation state and their complexes are generally back-bonded. | |||||||||
| Congeneric Series: | Few congeneric series, although periodicity is seen down groups:
|
SUMBER : http://www.meta-synthesis.com/webbook/12_lab/lab.html

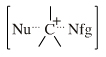
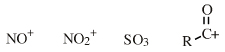
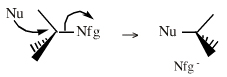
No comments:
Post a Comment